Sunday, 11 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
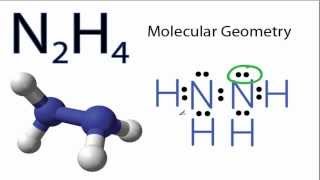
NO2 Molecular Geometry / Shape and Bond Angles (Note: exact bond angle is 134.3)
A quick explanation of the molecular geometry of NO2 including a description of the NO2 bond angles. Note the exact bond angle is 134.3 degrees).
Looking at the NO2 Lewis structure we can see that there are two atoms attached to the central Nitrogen (N) atom and that there is a single electron on the central N atom. Based on VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) the electron clouds on atoms and single electron around the N will repel each other. As a result they will be pushed apart giving the NO2 molecule a bent geometry or shape.
The NO2 bond angle will be about 109 degrees since it has a bent molecular geometry.
Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/
Теги:
Molecular Geometry Shape NO2 molecular geometry NO2 shape Molecular geometry of NO2 Molecular Shape of NO2 NO2 Bond Angles Bond Angles of NO2 Molecular Geometry for NO2 Molecular shape of NO2 Molecule shape of NO2 molecular geometry molecular shape VSPRE Valence Shell Pair Repulsion Theory
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5