Sunday, 11 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
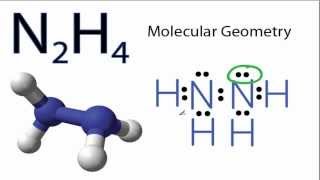
N2H4 Molecular Geometry and Bond Angles (actual bond angle is less than 109.5 degrees)
We'll determine the N2H4 molecular geometry with respect to the Nitrogen on the right (the other Nitrogen atom will have the same shape since they are symmetrical).
Looking at the N2H4 Lewis structure we can see that there are three atoms attached to the Nitrogen of interest and that there is one lone pair of electrons (on the N of interest). Based on VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) the electron clouds on the atoms (and lone pair) around the N will repel each other. As a result they will be pushed apart giving the trigonal pyramidal molecule a bent molecular geometry or shape.
The N2H4 bond angle will be about 109 degrees since it has a bent trigonal pyramidal geometry.
Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/
Теги:
trigonal pyramidal N2H4 molecular geometry N2H4 shape Molecular geometry of N2H4 Molecular Shape of N2H4 N2H4 Bond Angles Bond Angles of N2H4 Molecular Geometry for N2H4 Molecular shape of N2H4 Molecule shape of N2H4 molecular geometry molecular shape VSPRE Valence Shell Pair Repulsion Theory Molecular Geometry
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5