Wednesday, 14 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
ICl4- Lewis Structure - How to Draw the Lewis Structure for ICl4-
A step-by-step explanation of how to draw the ICl4- Lewis Structure
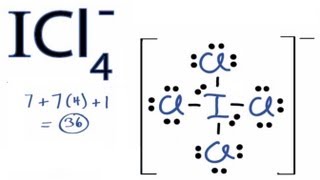
For the ICl4- Lewis structure the total number of valence electrons (found on the periodic table) for the ICl4- molecule. Once we know how many valence electrons there are in ICl4- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
In the Lewis structure of ICl4- there are total of 36 valence electrons.
Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons.
Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/
Теги:
ICl4- Lewis Structure Lewis Structure for ICl4- ICl4- ICl4- Electron Dot Structure Electron Dot Structure for ICl4- How to Draw the Lewis Structure for ICl4- How to Draw the Electron Dot Structure for ICl4- Lewis Structures Electron Dot Structures ICl4- Electron Dot Diagram Valence Electrons Chemistry Help Chemical Bonding Formal Charges Lewis structure of ICl4- ICl4 -1 Lewis structure Lewis structure for ICl4 -1 ICl4 1- Lewis structure
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5