Saturday, 17 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
Is C2H6 Polar or Non-polar? (Ethane)
Learn to determine if C2H6 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).
We start with the Lewis Structure and then use VSEPR to determine the shape of the molecule. After that we’ll look at how the shape of the molecule, based on VSEPR, allows us to determine if the entire molecule is polar or nonpolar.
If you look at the Lewis Structure for C2H6 it appears to be a symmetrical molecule. However, to determine if C2H6 is polar we consider the molecular geometry. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons.
While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like C2H6 these bonds are evenly distributed and cancel out. There is no net dipole and the C2H6 is non-polar.
Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Теги:
is C2H6 polar is C2H6 nonpolar is C2H6 polar or nonpolar is C2H6 a polar or nonpolar molecule polarity of C2H6 is ethane polar or nonpolar polar or nonpolar C2H6 C2H6 polar or nonpolar polarity polar or nonpolar determine if C2H6 polar is polar C2H6 is nonpolar C2H6 net dipole C2H6 C2H6 molecular geometry C2H6 molecular shape C2H6 VSEPR C2H6 valence electrons
Похожие видео
Мой аккаунт


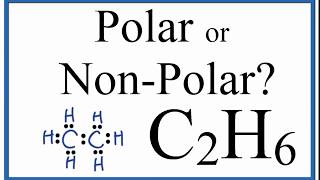 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5


