Wednesday, 14 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
Wittig Reaction for CSIR NET/ GATE/IIT JAM with many examples.
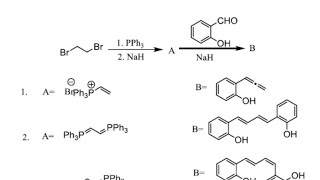
The Wittig reaction was discovered in 1954 by Georg Wittig, for which he was awarded the Nobel Prize in Chemistry in 1979. The Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide. It is widely used in organic synthesis for the preparation of alkenes. It should not be confused with the Wittig rearrangement. For the reaction with aldehydes, the double bond geometry is readily predicted based on the nature of the ylide. With unstabilised ylides (R3 = alkyl) this results in (Z)-alkene product with moderate to high selectivity. With stabilized ylides (R3 = ester or ketone), the (E)-alkene is formed with high selectivity. The (E)/(Z) selectivity is often poor with semistabilized ylides (R3 = aryl). Reaction is very important for CSIR NET/GATE/IIT JAM entrance examination. following are the important links discussed in the lecture:
https://www.youtube.com/watch?v=odnqNytqqLg&t=757s
Теги:
wittig reaction wittig olefination Julia olefination peterson olefination corey chaykovsky reaction tebbe olefination carbonyl compounds to alkene triphenyl phosphonium ylide triphenylphosphine oxide unstabilised ylides stabilized ylides
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5