Saturday, 17 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
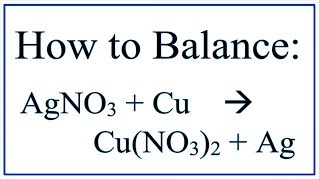
Silver Production from Silver Nitrate using a Copper Pipe
Here I show you how to recover silver from silver nitrate. I began by placing 130.5 grams of silver nitrate into 500 ml of distilled water. A copper pipe (cleaned first with a Dremel to remove the oxide coating) was suspended in the solution and left for 30 min. The silver nitrate reacts with copper to form crystals of silver metal and a blue solution of copper nitrate:
2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag
The first and last 30 seconds are in real time, the rest has been sped up to highlight the reaction. The precipitate is then filtered, washed several times with boiling water to remove the copper nitrate, then melted in a high-temp oven to produce 82.87 grams of 98-99% pure silver.
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5