Saturday, 17 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
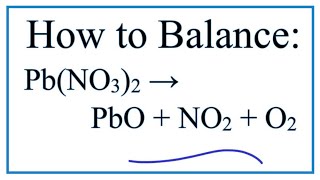
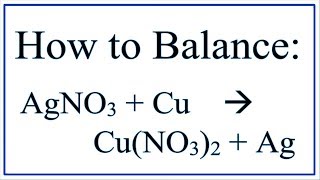
Balance AgNO3 + Cu = Cu(NO3)2 + Ag (Silver Nitrate and Copper)
In order to balance AgNO3 + Cu → Cu(NO3)2 + Ag you'll need to be sure to count all of Ag, N, O, and Cu Cl atoms on each side of the chemical equation. Once you know how many of each type of atom you have you can only change the coefficients (the numbers in front of atoms or compounds) in order to balance the equation.
The trick to balancing equations with polyatomic ions is to treat the ion as one item. For example, I have one NO3 on the reactants (left) side of the equation and two NO3's on the products (right) side. This makes the balancing vastly easier. This technique works for many chemical equations.
Drawing/writing done in InkScape on a Wacom Bamboo table. Screen capture done with Camtasia Studio 4.0. Done on a Microsoft Dell Dimension computer.
Теги:
How to Balance: AgNO3 + Cu → Cu(NO3)2 + Ag balancing AgNO3 + Cu → Cu(NO3)2 + Ag AgNO3 + Cu → Cu(NO3)2 + Ag balanced equation AgNO3 + Cu → Cu(NO3)2 + Ag balanced molecular equation Silver nitrate and copper balancing chemical equations balancing chemical reactions how to balance chemical equations balancing equations worksheet balancing of chemical equations balancing equations practice
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5