Tuesday, 13 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
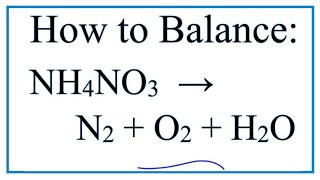
Net Ionic Equation for MgSO4 + BaCl2 (Magnesium sulfate and Barium chloride)
To write the net ionic equation for MgSO4 + BaCl2 we follow three main steps. First, we balance the molecular equation. Second, we break the soluble ionic compounds, the ones with an (aq) after them, into their ions. Finally, we cross out any spectator ions (ions that appear on both sides of the equation).
General Steps:
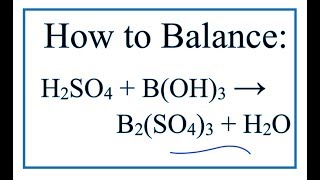
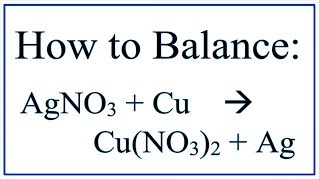
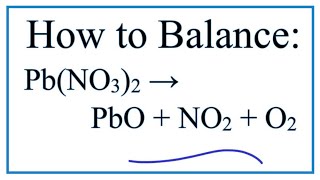
1. Write the balanced molecular equation.
2. Write the state (s, l, g, aq) for each substance.
3. Split soluble compounds into ions (the complete ionic equation).
4. Cross out the spectator ions on both sides of complete ionic equation.
5. Write the remaining substances as the net ionic equation.
More chemistry help at http://www.Breslyn.org
Теги:
breslyn Net ionic equation for MgSO4 + BaCl2 Net ionic equation for Magnesium sulfate + Barium chloride net ionic equation total ionic equation Magnesium sulfate and Barium chloride
Похожие видео
Мой аккаунт


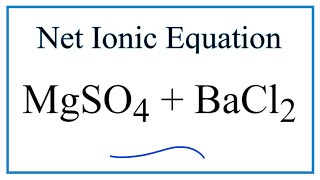 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5