Thursday, 15 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
XII Chemistry | p-Block 16 Group Elements | Chemical Properties Part-1 by Ruchi Ma'am
Class XII - Chemistry
Unit 07 - p-Block 16 Group Elements
Chemical Properties Part-1
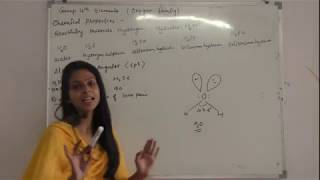
Oxygen Most Reactive, Reactivity Towards Hydrogen - Formation of Compounds like H2F, Comparative Study of their Shapes, Bond Angles, Volatility etc.
Reactivity Towards Oxygen Formation of di- and tri-oxides
This video is about some chemical properties of group 16 elements. All these react with hydrogen to form hydrides. Acidic character and reducing character of these hydrides increase down the group but thermal stability decrease down the group. Group 16 elements react with oxygen to form two types of oxides - dioxide and trioxide . These oxide are acidic and down the group acidic character decreases.
This Channel initiated by Dr. D. R. Vij (former Professor of Physics, Kurukshetra University, India) primarily aims at taking free education to the doorsteps of learners who for various economic and social factors can not avail benefits of regular coaching.
In view of the congested classrooms coupled with hefty tuition fee, social taboos and hazards of travelling (for girls in particular) this mode of extending education had become a necessity.
This self-learning approach is a definite step towards democratisation of education i.e. taking education even to individuals who staying in remote and far off areas were being deprived of education for no fault of theirs.
Viewers are requested to click LIKE button if they like a video and SUBSCRIBE to this channel to get notifications of more video lessons being uploaded from time to time in future. They are also requested to SHARE videos with nears and dears who can be benefitted.
All video lessons are systematically indexed on the website: www.gianmandir.com wherein one can register free of cost. All videos are also available through our “Gianmandir” APP which can be downloaded from Google Play Store.
With thanks
Dr. D. R. Vij
Professor of Physics (Retd.)
(Mob) +919416220316
Теги:
XII Chemistry p-Block 16 Group Elements Chemical Properties Oxygen Most Reactive Reactivity Towards Hydrogen - Formation of Compounds like H2F Comparative Study of their Shapes Bond Angles Vola
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5