Tuesday, 13 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
Type of Reaction for Mg + O2 = MgO
In this video we determine the type of chemical reaction for the equation Mg + O2 = MgO (Magnesium + Oxygen gas). Note that this reaction is sometimes also called Combustion as O2 is involved.
Since we have a two substances combining this is a Synthesis Reaction (also called a Combination Reaction” reaction). Synthesis reactions follow the general form of:
A + B → AB
An example of a synthesis reaction is: Fe + S → FeS or Na + Cl2 → NaCl .
There is no specific number of reactants in a combination reaction.
---Type of Reactions---
Synthesis (Combination): A + B → AB
Decomposition: AB → A + B
Single Replacement : A + BC → B + AC
Double Replacement: AB + CD → AD + BC
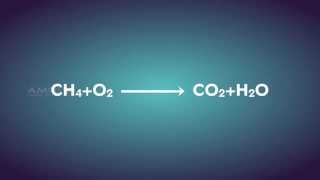
Combustion: CxHy + O2 → CO2 + H2O
Neutralization: HX + MOH → MX + H2O
Reduction-Oxidation (Redox): Electrons are exchanged. Many of the above reactions are redox
Being able to determine the type of reaction by looking at reactants can help in predicting the outcome of the chemical reaction.
Теги:
breslyn Type of reaction Mg + O2 = MgO type of reaction Magnesium + Oxygen gas reaction type Mg + O2 = MgO what type of reaction is Mg + O2 = MgO Mg + O2 = MgO combination reaction combination synthesis reaction kind of reaction Mg + O2 = MgO
Похожие видео
Мой аккаунт


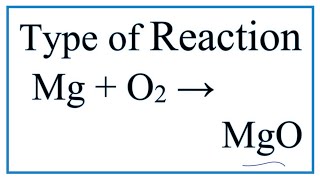 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5