Wednesday, 14 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
Molar Mass From Osmotic Pressure - Molarity & Van't Hoff Factor - Chemistry Problems
This chemistry video tutorial explains how to calculate the molar mass from osmotic pressure. Given the osmotic pressure and the van't hoff factor, you need to calculate the molarity of the solution which can help you to determine the number of moles of solute in the solution if you know the volume of the solution. The molar mass is the ration between the mass in grams and number of moles of solute.
New Chemistry Video Playlist:
https://www.youtube.com/watch?v=bka20Q9TN6M&t=25s&list=PL0o_zxa4K1BWziAvOKdqsMFSB_MyyLAqS&index=1
Access to Premium Videos:
https://www.patreon.com/MathScienceTutor
Facebook: https://www.facebook.com/MathScienceTutoring/
Теги:
molar mass from osmotic pressure molar mass molarity osmotic pressure van't hoff factor chemistry practice problems molar mass determination problems solute solution mass grams moles
Похожие видео
Мой аккаунт


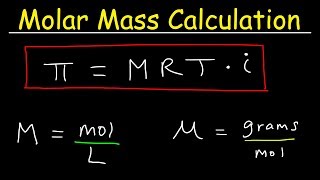 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5