Monday, 19 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
How to find the Oxidation Number for Fe in Fe2O3
To find the correct oxidation number for Fe2O3 (Iron (III) oxide), and each element in the compound, we use a few rules and some simple math.
First, since the Fe2O3 doesn’t have an overall charge (like NO3- or H3O+) we could say that the total of the oxidation numbers for Fe2O3 will be zero since it is a neutral compound.
We write the oxidation number (O.N.) for elements that we know and use these to figure out oxidation number for Fe.
----------
GENERAL RULES
In a netural compound all O.N. must add up to zero.
Group 1 = +1
Group 2 = +2
Hydrogen with Non-Metals = +1
Hydrogen with Metals (or Boron) = -1
Fluorine = -1
Oxygen = -2 (except in H2O2 or with Fluorine)
Group 17(7A) = -1 except with Oxygen and other halogens lower in the group
----------
We know that Oxygen usually is -2 with a few exceptions. When Oxygen is in a peroxide, like H2O2 (Hydrogen peroxide), it has a charge of -1. When it is bonded to Fluorine (F) it has an oxidation number of +2.
Here it is bonded to element symbol so the oxidation number on Oxygen is -2. Using this information we can figure out the oxidation number for the element Fe in Fe2O3.
Теги:
breslyn Oxidation number of Fe2O3 oxidation numbers Fe2O3 oxidation number Iron (III) oxide Iron (III) oxide oxidation number oxidation number oxidation number element Fe oxidation number element Fe in Fe2O3 how to find the oxidation number for Iron (III) oxide how to find the oxidation number for Fe2O3 what is the oxidation number for Fe2O3 what is the oxidation number for Iron (III) oxide what is the oxidation number of Fe2O3
Похожие видео
Мой аккаунт


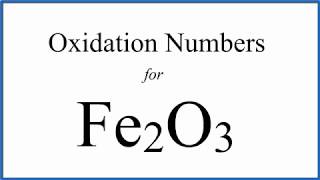 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5


