Tuesday, 13 January, 2026г.
















Где искать: по сайтам Запорожской области, статьи, видео ролики
пример: покупка автомобиля в Запорожье
Allotropes of Carbon - Class 10 Tutorial
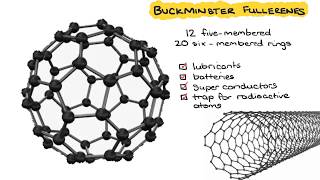
Allotropes are different physical forms of the same element. All elements are made up uniquely of their own atoms and therefore any physical differences must be a consequence of how the atoms are joined together - their arrangement within the bulk structure. Many elements exhibit allotropy as there are often varous ways in which the atoms can be linked together into molecules and also different ways in which the molecules can be arranged to make larger structures. The two most common allotropes of carbon are diamond and graphite. The crystal structure of diamond is an infinite three-dimensional array of carbon atoms, each of which forms a structure in which each of the bonds makes equal angles with its neighbours. The crystal structure of graphite amounts to a parallel stacking of layers of carbon atoms.
Теги:
create@amrita amrita university amrita viswa vidyapeetham research allotropes diamond graphite carbon atomic structure elements crystal structure atoms
Похожие видео
Мой аккаунт


 У вашего броузера проблема в совместимости с HTML5
У вашего броузера проблема в совместимости с HTML5